Active Anode Materials Manufacturing
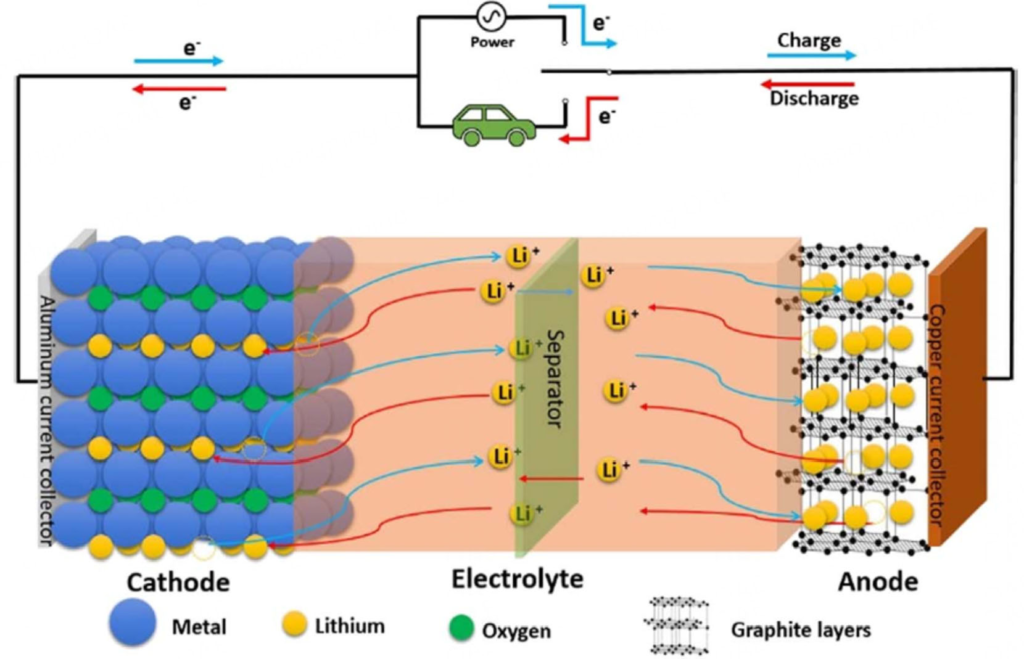
Active Anode materials (AAM) are the materials in a battery’s anode that store and release lithium ions during charge and discharge. By manufacturing AAM from both natural graphite (derived from the Graphite Creek mine) and artificial graphite, Graphite One intends to complete America’s graphite materials supply chain.
Source: Benchmark Mineral Intelligence
Graphite is available in two forms: natural graphite (“NG”) and artificial graphite (“AG”). Both must be further processed to convert each into AAM to effectively function in lithium-ion batteries. AAM is designed and manufactured to meet each customer’s specifications. The AAM design necessary to each particular specification required selection from a range of primary component combinations of NG AAM and AG AAM, either separately or in blends, and sometimes with other materials such as silicon and SiO. The specifications can generally be categorized to attain fast charging, high energy density, or longer life cycles.
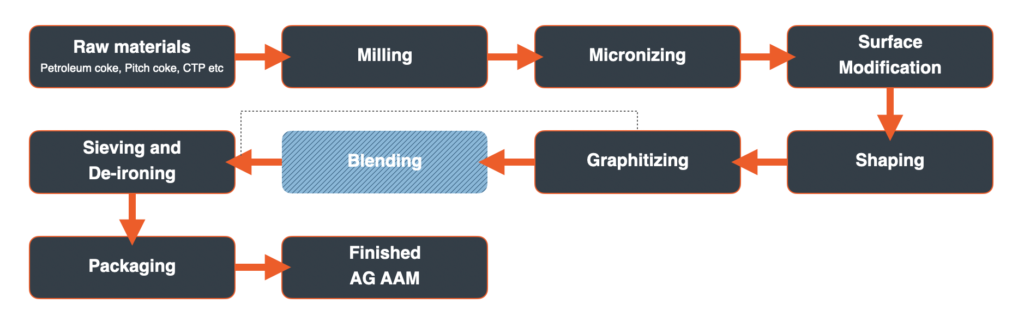
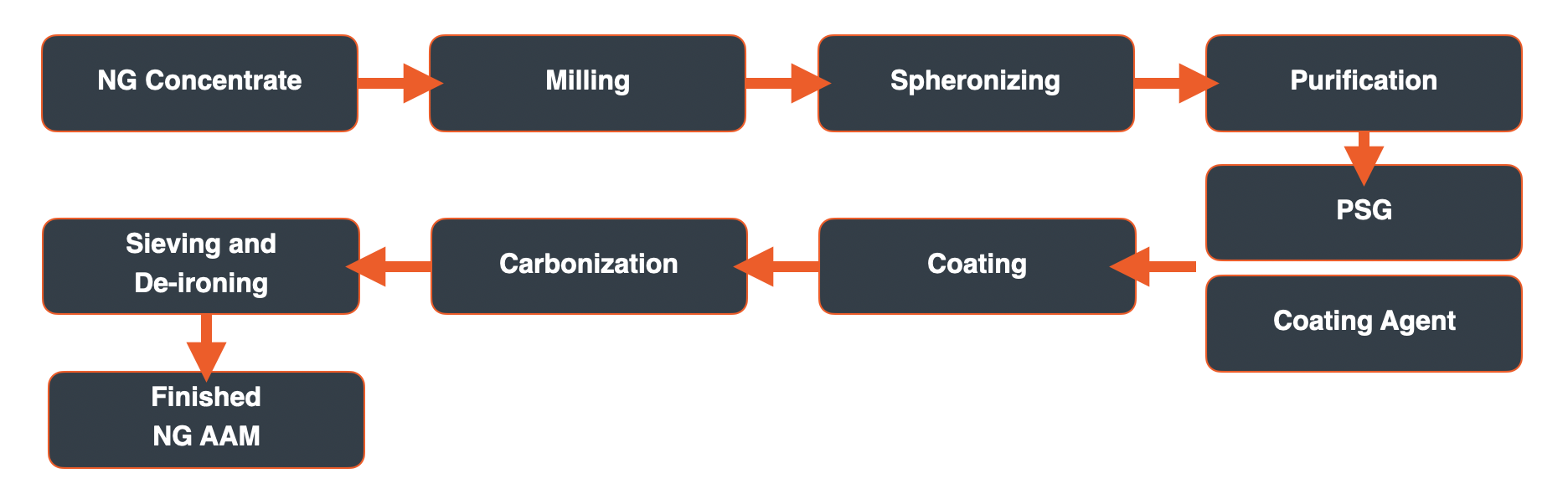
Graphite One plans to make both AG AAM and NG AAM for the electric vehicle and energy storage systems markets. The process for making each can generally be separated into three phases: preparation of the precursor materials, graphitization or purification of the precursor materials, and finishing/blending of the graphitized or purified material to produce the final AAM product. The precursor materials for AG AAM include petroleum cokes and pitches. For NG AAM, natural graphite concentrate and pitches. The following schematics generally describe the process for producing each.
Natural Graphite
Artificial Graphite